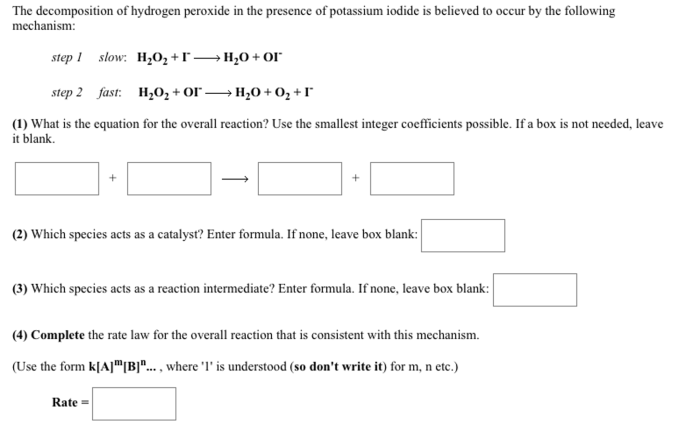
The analysis of hydrogen peroxide lab answers is a crucial aspect of scientific research and various industrial applications. Hydrogen peroxide, a versatile chemical compound, finds widespread use in numerous fields, and its precise analysis is essential to ensure accuracy and reliability in experimentation and practical implementations.
This comprehensive guide delves into the methods, procedures, and applications of hydrogen peroxide analysis, providing a thorough understanding of the subject matter.
Analysis of Hydrogen Peroxide: Analysis Of Hydrogen Peroxide Lab Answers

Hydrogen peroxide (H2O2) is a colorless liquid with a pungent odor. It is a powerful oxidizing agent and is used in a variety of applications, including bleaching, disinfection, and rocket propulsion. The analysis of hydrogen peroxide is important in various fields, including environmental monitoring, industrial processes, and healthcare.
Methods of Hydrogen Peroxide Analysis
- Titration: This method involves reacting hydrogen peroxide with a known concentration of a reducing agent, such as potassium permanganate or sodium thiosulfate. The endpoint of the titration is reached when the reducing agent has completely reacted with the hydrogen peroxide.
- Spectrophotometry: This method involves measuring the absorbance of hydrogen peroxide at a specific wavelength. The absorbance is directly proportional to the concentration of hydrogen peroxide in the sample.
- Enzymatic assays: These methods use enzymes that catalyze the reaction of hydrogen peroxide with a specific substrate. The rate of the reaction is proportional to the concentration of hydrogen peroxide in the sample.
Procedure for Hydrogen Peroxide Analysis Using Titration
- Prepare a standard solution of potassium permanganate or sodium thiosulfate.
- Pipette a known volume of the hydrogen peroxide sample into a flask.
- Add the standard solution to the flask until the endpoint of the titration is reached.
- Calculate the concentration of hydrogen peroxide in the sample using the following equation:
Concentration of H2O2 = (Volume of standard solution x Concentration of standard solution) / Volume of sample
Interpretation of Results, Analysis of hydrogen peroxide lab answers
The results of a hydrogen peroxide analysis can be used to determine the concentration of hydrogen peroxide in a sample. The accuracy and precision of the results can be affected by a number of factors, including the method of analysis, the quality of the reagents, and the skill of the analyst.
Applications of Hydrogen Peroxide Analysis
- Environmental monitoring: Hydrogen peroxide is a pollutant that can be harmful to aquatic life. Hydrogen peroxide analysis is used to monitor the levels of hydrogen peroxide in water and soil.
- Industrial processes: Hydrogen peroxide is used in a variety of industrial processes, including bleaching, disinfection, and wastewater treatment. Hydrogen peroxide analysis is used to control the concentration of hydrogen peroxide in these processes.
- Healthcare: Hydrogen peroxide is used as an antiseptic and disinfectant. Hydrogen peroxide analysis is used to ensure that the concentration of hydrogen peroxide in healthcare products is safe for use.
Safety Considerations
Hydrogen peroxide is a corrosive and oxidizing agent. It can cause skin burns and eye damage. It is important to wear gloves and eye protection when working with hydrogen peroxide. Hydrogen peroxide should be stored in a cool, dark place away from flammable materials.
Questions and Answers
What is the purpose of hydrogen peroxide analysis in laboratory settings?
Hydrogen peroxide analysis is conducted to determine the concentration or presence of hydrogen peroxide in various samples. It is commonly used in research, quality control, and industrial processes.
What are the different methods used to analyze hydrogen peroxide concentration?
Several methods are employed to analyze hydrogen peroxide concentration, including titration, spectrophotometry, and enzymatic assays. Each method utilizes specific techniques and reagents to measure the amount of hydrogen peroxide present.
How do you interpret the results of a hydrogen peroxide analysis?
The results of a hydrogen peroxide analysis are typically expressed as concentration units, such as molarity or percentage. Factors that can affect the accuracy and precision of the results include the method used, sample preparation, and environmental conditions.