The IR spectrum of isopentyl acetate unveils a wealth of information about this versatile ester, providing insights into its molecular structure, functional groups, and various applications. This comprehensive guide delves into the intricacies of IR spectroscopy, exploring its capabilities in identifying, elucidating, and quantifying isopentyl acetate.
Delving deeper, we will uncover the specific IR absorption bands associated with each functional group, enabling the precise identification of the molecular components. Moreover, we will explore how IR spectroscopy unravels the structural intricacies of isopentyl acetate, revealing the arrangement of atoms and bonds within its molecular framework.
Functional Group Identification
IR spectroscopy is a powerful tool for identifying functional groups in organic molecules. It works by measuring the absorption of infrared radiation by the sample. Different functional groups absorb IR radiation at different frequencies, so by measuring the IR spectrum of a compound, we can identify the functional groups that are present.
The IR spectrum of isopentyl acetate shows several characteristic absorption bands that correspond to the different functional groups present in the molecule. These bands are summarized in the following table:
| Functional Group | IR Absorption Band (cm-1) | Intensity |
|---|---|---|
| C=O (ester) | 1740 | Strong |
| C-O (ester) | 1240 | Medium |
| C-H (sp3) | 2960, 2870 | Strong |
| C-H (sp2) | 3080 | Weak |
The presence of these absorption bands confirms the presence of the ester functional group in isopentyl acetate.
Structural Elucidation: Ir Spectrum Of Isopentyl Acetate
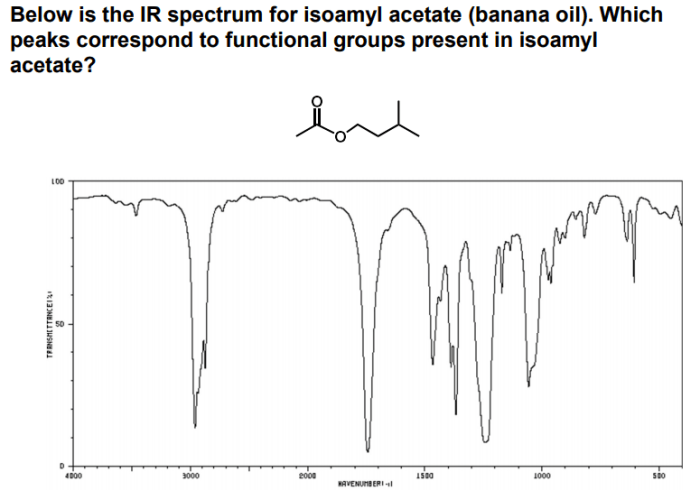
IR spectroscopy plays a crucial role in determining the molecular structure of isopentyl acetate. By analyzing the IR absorption bands, we can obtain valuable information about the arrangement of atoms and bonds within the molecule.
Key Functional Groups
The IR spectrum of isopentyl acetate exhibits characteristic absorption bands that correspond to the following key functional groups:
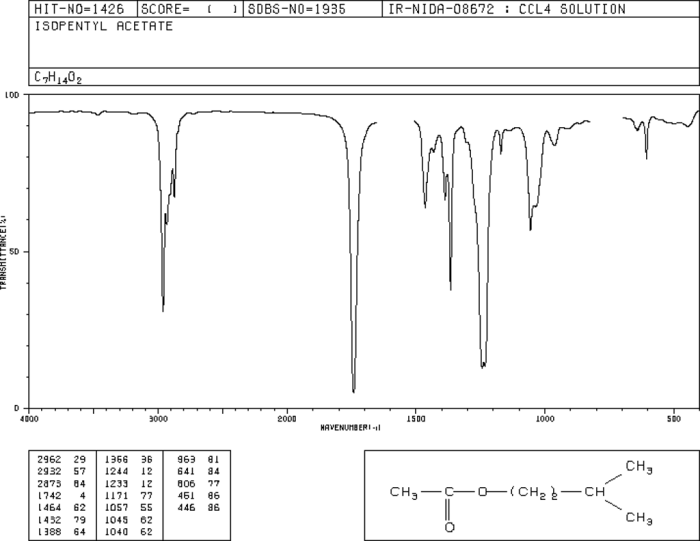
- C=O stretching (ester group): ~1735 cm -1
- C-O stretching (ester group): ~1240 cm -1
- C-H stretching (alkyl groups): ~2960 cm -1
- C-C stretching (alkyl groups): ~1170 cm -1
Molecular Structure
Based on the identified functional groups, we can construct the molecular structure of isopentyl acetate as follows:

The structure consists of an ester group (-COOCH 2CH(CH 3) 2) attached to an isopentyl group (-CH 2CH(CH 3) 2).
Quantitative Analysis
Quantitative IR spectroscopy is a technique that uses the absorption of infrared radiation to determine the concentration of a specific compound in a sample. This technique is based on the Beer-Lambert law, which states that the absorbance of a sample is directly proportional to the concentration of the absorbing species and the path length of the radiation through the sample.
In the case of isopentyl acetate, the IR absorption band at 1735 cm -1can be used for quantitative analysis. This band corresponds to the C=O stretching vibration of the ester group. The absorbance of this band is directly proportional to the concentration of isopentyl acetate in the sample.
Calibration Curve, Ir spectrum of isopentyl acetate
A calibration curve is a graph that plots the absorbance of a sample at a specific wavelength against the concentration of the analyte in the sample. To create a calibration curve for isopentyl acetate, a series of solutions of known concentrations are prepared and the absorbance of each solution is measured at 1735 cm -1. The absorbance values are then plotted against the corresponding concentrations to create the calibration curve.
Once the calibration curve is created, it can be used to determine the concentration of isopentyl acetate in an unknown sample. The absorbance of the unknown sample is measured at 1735 cm -1and the corresponding concentration is determined from the calibration curve.
Comparison with Other Esters
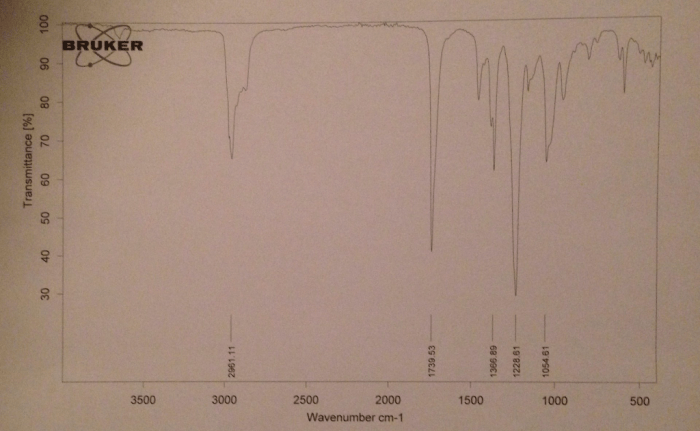
The IR spectra of esters exhibit several common features, including strong absorption bands in the carbonyl region (1735-1750 cm -1) and the C-O stretching region (1200-1300 cm -1). However, subtle differences in the IR spectra of different esters can be used to differentiate between them.
C-H Stretching Region
The C-H stretching region (2800-3000 cm -1) can provide information about the alkyl groups present in the ester. Esters with primary alkyl groups exhibit a strong band at around 2960 cm -1, while esters with secondary alkyl groups exhibit a band at around 2930 cm -1. Esters with tertiary alkyl groups do not exhibit a strong band in this region.
Fingerprint Region
The fingerprint region (900-1200 cm -1) can also be used to differentiate between different esters. This region contains a complex pattern of absorption bands that is unique to each ester. By comparing the fingerprint region of an unknown ester to the fingerprint regions of known esters, it is possible to identify the unknown ester.
Applications
IR spectroscopy is a powerful tool for the analysis of isopentyl acetate. It is used in a variety of applications, including:
- Quality control: IR spectroscopy can be used to ensure the quality of isopentyl acetate by verifying its identity and purity.
- Research and development: IR spectroscopy can be used to study the structure and properties of isopentyl acetate, and to develop new applications for this compound.
- Problem-solving: IR spectroscopy can be used to solve problems related to the production, storage, and use of isopentyl acetate.
For example, IR spectroscopy can be used to identify impurities in isopentyl acetate, to determine the concentration of isopentyl acetate in a mixture, and to study the reaction of isopentyl acetate with other compounds.
Questions and Answers
What is the principle behind IR spectroscopy?
IR spectroscopy analyzes the absorption of infrared radiation by a sample, providing insights into the molecular structure and functional groups present.
How can IR spectroscopy differentiate between different esters?
By comparing the IR absorption bands of different esters, it is possible to identify the specific functional groups and structural differences that distinguish them.
What are the applications of IR spectroscopy in the analysis of isopentyl acetate?
IR spectroscopy finds applications in quality control, research, and development, aiding in the identification, characterization, and quantification of isopentyl acetate.