History of an atom worksheet answers provides an in-depth exploration of the fundamental concepts surrounding the structure and behavior of atoms, the basic units of matter. This comprehensive guide delves into the historical milestones, key scientific figures, and experimental discoveries that have shaped our understanding of the atomic realm.
From the pioneering work of Democritus to the groundbreaking experiments of Rutherford, Bohr, and Schrödinger, this resource traces the evolution of atomic theory, shedding light on the intricate structure of atoms, including the nucleus, electrons, and energy levels.
History of the Atomic Model
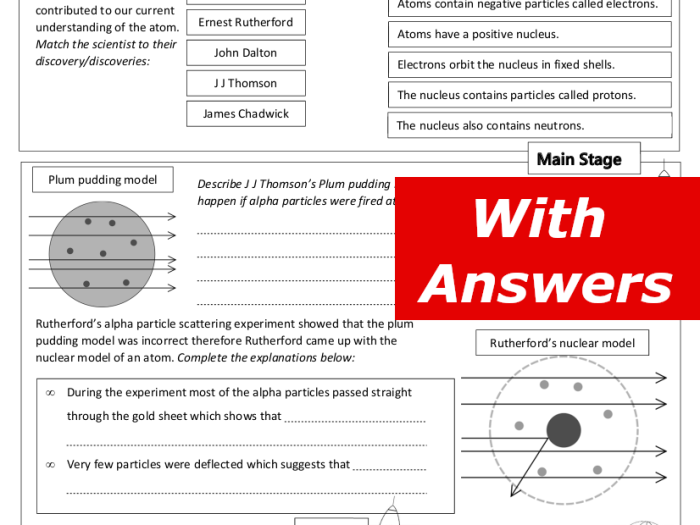
The atomic model has undergone significant evolution since the early philosophical ideas about matter. The concept of atoms as indivisible building blocks of matter has been refined over centuries, leading to the modern understanding of the atom as a complex structure with a nucleus and surrounding electrons.
Key Discoveries and Scientists
- 400 BC:Democritus proposed the idea of atoms as indivisible particles.
- 1803:John Dalton’s atomic theory provided the first scientific basis for the atomic model.
- 1897:J.J. Thomson discovered the electron, demonstrating that atoms were not indivisible.
- 1911:Ernest Rutherford’s gold foil experiment revealed the existence of a small, dense nucleus.
- 1913:Niels Bohr proposed a model of the atom with electrons orbiting the nucleus in specific energy levels.
- 1926:Erwin Schrödinger developed the quantum mechanical model of the atom, which describes electrons as waves with specific probabilities of being found in certain regions.
Structure of the Atom

An atom is the fundamental unit of matter and the building block of all chemical elements. It consists of a central nucleus surrounded by electrons.
Nucleus
The nucleus is the central core of the atom and contains positively charged protons and neutral neutrons. The number of protons in the nucleus determines the element’s atomic number, which identifies the element. The number of neutrons determines the isotope of the element.
Electrons
Electrons are negatively charged particles that orbit the nucleus in specific energy levels. The energy levels are arranged in shells and subshells, with each shell holding a specific number of electrons.
Energy Levels
Electrons occupy specific energy levels based on their energy. The lowest energy level is the first shell, which can hold up to two electrons. The second shell can hold up to eight electrons, and so on.
Atomic Number and Mass Number
The atomic number of an element is the number of protons in its nucleus, which is equal to the number of electrons in a neutral atom. The mass number of an element is the total number of protons and neutrons in its nucleus.
Orbital Diagrams
Orbital diagrams are visual representations of the distribution of electrons in an atom’s energy levels. Each orbital represents a specific energy level and subshell and can hold up to two electrons.
Properties of Elements
The properties of elements are determined by their atomic structure, particularly the arrangement of electrons in energy levels. Elements can be classified into various categories based on their properties, including metals, nonmetals, and noble gases.
Periodic Trends in Atomic Properties, History of an atom worksheet answers
The periodic table organizes elements based on their atomic number, which determines the number of electrons in an atom. As we move across a period (row) of the periodic table, the number of electrons in the outermost energy level increases.
This leads to periodic trends in atomic properties, such as electronegativity and ionization energy.
- Electronegativityis the ability of an atom to attract electrons in a chemical bond. It generally increases from left to right across a period and decreases from top to bottom within a group.
- Ionization energyis the energy required to remove an electron from an atom. It generally increases from left to right across a period and decreases from top to bottom within a group.
Arrangement of Electrons and Chemical Reactivity
The arrangement of electrons in energy levels influences the chemical reactivity of an element. Elements with unpaired electrons in their outermost energy level are more reactive than those with all electrons paired. This is because unpaired electrons can participate in chemical reactions to form bonds with other atoms.
Example:Sodium (Na) has one unpaired electron in its outermost energy level, making it a highly reactive metal. In contrast, helium (He) has all its electrons paired, making it a very unreactive noble gas.
Chemical Bonding: History Of An Atom Worksheet Answers
Chemical bonding refers to the forces that hold atoms together to form molecules and compounds. These bonds determine the physical and chemical properties of substances.
The stability of compounds depends on the strength of the bonds between their constituent atoms. Stronger bonds lead to more stable compounds.
Types of Chemical Bonds
Ionic Bonds
Ionic bonds are formed between atoms of metals and non-metals. In an ionic bond, one atom transfers one or more electrons to another atom, creating positively and negatively charged ions.
For example, sodium (Na) and chlorine (Cl) can form an ionic bond. Sodium loses one electron to chlorine, becoming a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-).
Covalent Bonds
Covalent bonds are formed between atoms of non-metals. In a covalent bond, atoms share one or more pairs of electrons.
For example, hydrogen (H) and chlorine (Cl) can form a covalent bond by sharing a pair of electrons. This forms a stable molecule of hydrogen chloride (HCl).
Metallic Bonds
Metallic bonds are formed between atoms of metals. In a metallic bond, the metal atoms share their valence electrons in a “sea of electrons.”
The valence electrons are not attached to any particular atom, allowing metals to conduct electricity and heat well.
Nuclear Reactions

Nuclear reactions involve changes in the atomic nuclei, resulting in the release or absorption of enormous amounts of energy. These reactions play a crucial role in various fields, including energy production and medicine.
Two prominent types of nuclear reactions are nuclear fission and nuclear fusion. Nuclear fission involves the splitting of heavy atomic nuclei into smaller ones, while nuclear fusion combines lighter nuclei into heavier ones.
Radioactivity
Radioactivity refers to the spontaneous emission of particles or energy from unstable atomic nuclei. This decay process leads to the formation of a more stable nucleus. The rate of radioactive decay is characterized by its half-life, which is the time it takes for half of the radioactive atoms in a sample to decay.
Applications of Nuclear Reactions
- Energy Production:Nuclear fission is utilized in nuclear power plants to generate electricity. The controlled release of energy from fission reactions provides a reliable and efficient source of power.
- Medical Applications:Nuclear reactions are employed in medical imaging techniques such as PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography). These techniques help diagnose and monitor various medical conditions.
- Cancer Treatment:Radiation therapy uses high-energy radiation from nuclear reactions to target and destroy cancerous cells.
Expert Answers
What is the significance of Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment provided crucial evidence for the existence of a small, dense nucleus within the atom, revolutionizing our understanding of atomic structure.
How did Bohr’s model contribute to atomic theory?
Bohr’s model introduced the concept of quantized energy levels, explaining the emission and absorption spectra of atoms and providing a more accurate description of atomic structure.
What is the role of electrons in chemical bonding?
Electrons play a fundamental role in chemical bonding, determining the chemical properties and reactivity of atoms by forming ionic, covalent, or metallic bonds.