The Lewis dot structure of uranium, a captivating topic in chemistry, provides a gateway into understanding the intriguing world of this element. This structure unveils the intricate arrangement of electrons around the uranium atom, shedding light on its unique properties and behavior.
Uranium’s Lewis dot structure unveils its valence electrons, offering insights into its chemical reactivity and interactions with other elements. The octet rule, a guiding principle in chemistry, plays a crucial role in shaping uranium’s bonding tendencies, further enriching our comprehension of this remarkable element.
Lewis Dot Structure of Uranium
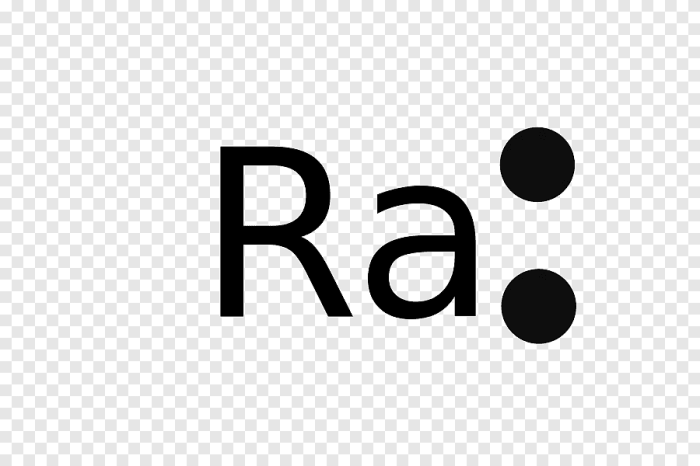
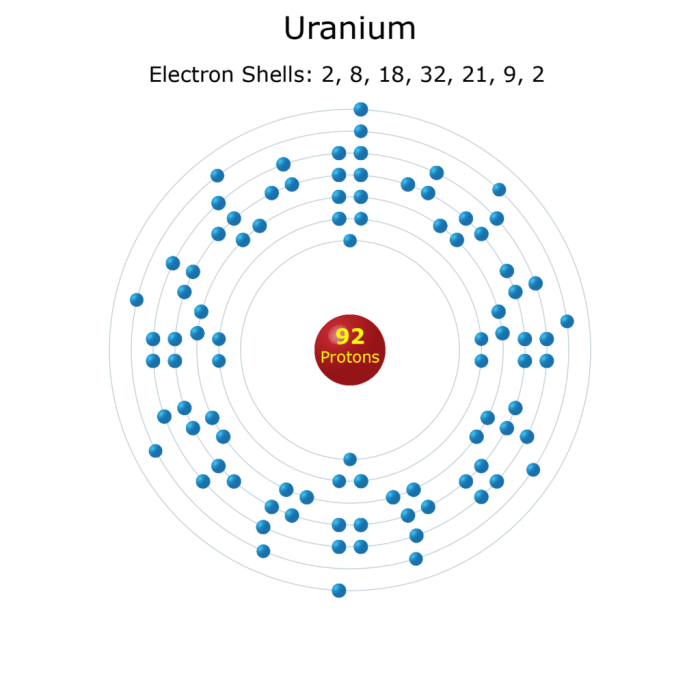
Uranium is a radioactive element with the atomic number 92. It is a heavy metal with a silvery-white appearance. Uranium is used as a fuel in nuclear reactors and is also used in the production of nuclear weapons.The Lewis dot structure of uranium shows the arrangement of the valence electrons in the atom.
Valence electrons are the electrons in the outermost energy level of an atom, and they determine the chemical properties of the element. Uranium has six valence electrons.The Lewis dot structure of uranium is as follows:“` . : . : U : . ‘ : ‘“`The six dots around the uranium atom represent the six valence electrons.
Uranium, an element with a fascinating atomic structure, exhibits a distinct Lewis dot structure. Its intricate arrangement of electrons provides insights into its chemical properties. To enhance our understanding of uranium’s electronic configuration, exploring the wordly wise book 7 lesson 11 can offer valuable supplementary information.
This lesson delves into the intricacies of Lewis dot structures, providing a deeper comprehension of uranium’s electronic behavior and its implications in various chemical reactions.
The uranium atom has a total of 92 electrons, but only the six valence electrons are shown in the Lewis dot structure.The octet rule states that atoms are most stable when they have eight valence electrons. Uranium does not follow the octet rule because it has six valence electrons.
However, uranium is still a stable element because it has a full f-orbital.
Properties of Uranium: Lewis Dot Structure Of Uranium
Uranium is a dense, silvery-white metal that is slightly radioactive. It is the heaviest naturally occurring element and is found in small amounts in uranium ores. Uranium is a poor conductor of electricity and heat and is highly reactive.Uranium has a variety of oxidation states, including +3, +4, +5, and +6. The most common oxidation states are +4 and +6. Uranium(IV) compounds are generally more stable than uranium(VI) compounds.
Uranium is a reactive metal and can react with a variety of elements, including oxygen, hydrogen, nitrogen, and carbon.
Physical Properties
* Uranium is a dense, silvery-white metal.
- It is the heaviest naturally occurring element.
- Uranium is a poor conductor of electricity and heat.
- It is slightly radioactive.
Chemical Properties, Lewis dot structure of uranium
* Uranium is a reactive metal.
- It can react with a variety of elements, including oxygen, hydrogen, nitrogen, and carbon.
- Uranium has a variety of oxidation states, including +3, +4, +5, and +6.
- The most common oxidation states are +4 and +6.
Uses of Uranium
Uranium is a versatile element with a wide range of applications, primarily in the nuclear industry. Its unique properties make it an essential component in various technologies.
Nuclear Power Plants
The primary use of uranium is in nuclear power plants, where it serves as the fuel for generating electricity. Uranium-235, a fissile isotope, undergoes a controlled nuclear fission process, releasing a tremendous amount of energy. This energy is harnessed to heat water, producing steam that drives turbines and generates electricity.
Nuclear Weapons
Uranium-235 and uranium-238 are also used in the production of nuclear weapons. When these isotopes undergo rapid nuclear fission, they release an immense burst of energy, creating a devastating explosion. The controlled release of this energy in nuclear power plants differs significantly from the uncontrolled reaction in nuclear weapons.
Other Applications
- Medical Imaging:Uranium-235 is used in medical imaging techniques like X-rays and gamma scans.
- Radiation Therapy:Uranium-238 is employed in radiation therapy to treat certain types of cancer.
- Scientific Research:Uranium isotopes are used in various scientific research applications, including nuclear physics and radioisotope dating.
Isotopes of Uranium
Uranium has 25 known isotopes, ranging from uranium-217 to uranium- 242. However, only three of these isotopes occur naturally: uranium-234, uranium-235, and uranium-238.
The stability and abundance of uranium isotopes vary significantly. Uranium-238 is the most stable and abundant isotope, accounting for over 99% of natural uranium. It has a half-life of 4.468 billion years, which is comparable to the age of the Earth.
Uranium-235 is the fissile isotope used in nuclear reactors and weapons. It is much less abundant than uranium-238, making up only about 0.72% of natural uranium. Uranium-235 has a half-life of 703.8 million years.
Uranium-234 is a decay product of uranium-238. It is also less abundant than uranium-238, accounting for about 0.0054% of natural uranium. Uranium-234 has a half-life of 245,500 years.
Applications of Uranium Isotopes
The different isotopes of uranium have unique applications based on their properties:
- Uranium-238:Used as fuel in nuclear reactors for electricity generation. It is also used in the production of plutonium-239, which is used in nuclear weapons.
- Uranium-235:Used as fuel in nuclear reactors and nuclear weapons. It is the only fissile isotope of uranium that can sustain a chain reaction.
- Uranium-234:Used in nuclear dating techniques and as a tracer in environmental studies.
Comparison with Other Elements
Uranium, an actinide element, exhibits similarities and differences in its Lewis dot structure and properties compared to other elements in the actinide series.
Lewis Dot Structure
The Lewis dot structure of uranium, like other actinides, features a central uranium atom surrounded by valence electrons. The number of valence electrons varies across the series, influencing the overall structure and properties of the elements.
Properties
- Atomic Size:Uranium, like other actinides, exhibits a gradual increase in atomic size as we move down the series. This is attributed to the addition of electron shells.
- Ionization Energy:Uranium’s first ionization energy is relatively low compared to other actinides. This is because of the large atomic size and the presence of loosely bound valence electrons.
- Oxidation States:Uranium, similar to other actinides, exhibits multiple oxidation states. The most common oxidation states for uranium are +3, +4, +5, and +6.
- Chemical Reactivity:Uranium is a highly reactive element, like other actinides. It readily forms compounds with various elements, including oxygen, halogens, and nitrogen.
- Radioactivity:All uranium isotopes are radioactive, a characteristic shared among actinides. The most stable isotope, uranium-238, has a half-life of 4.5 billion years.
Trends Across the Actinide Series
As we move across the actinide series, several trends in properties become apparent:
- Atomic Size:Atomic size generally increases from thorium to lawrencium.
- Ionization Energy:Ionization energy decreases down the series due to the increasing atomic size and loosely bound valence electrons.
- Oxidation States:The range of oxidation states exhibited by actinides increases as we move down the series.
- Chemical Reactivity:Actinides become more reactive as we move down the series, with elements like uranium and plutonium being highly reactive.
- Radioactivity:All actinides are radioactive, with half-lives varying depending on the isotope.
Health and Safety Considerations
Uranium poses significant health risks due to its radioactivity and potential for toxicity.
Prolonged exposure to uranium can lead to serious health issues, including cancer, kidney damage, and reproductive problems.
Safety Measures
To minimize these risks, strict safety measures are implemented when handling and storing uranium.
- Workers are required to wear protective gear, including respirators and gloves.
- Uranium is stored in shielded containers to prevent radiation exposure.
- Regular monitoring of air and water quality is conducted to ensure compliance with safety standards.
Regulations
The use of uranium is heavily regulated by government agencies worldwide.
- Regulations cover mining, processing, transportation, and disposal of uranium.
- These regulations aim to protect workers, the public, and the environment from potential hazards.
- Compliance with these regulations is mandatory and subject to regular inspections.
Popular Questions
What is the significance of the Lewis dot structure for uranium?
The Lewis dot structure of uranium provides a visual representation of the arrangement of valence electrons around the uranium atom, offering insights into its chemical behavior and bonding tendencies.
How does the octet rule apply to uranium?
Uranium typically follows the octet rule, which states that atoms tend to gain or lose electrons until they have eight valence electrons, resulting in a stable electron configuration.
What are the potential hazards associated with uranium exposure?
Uranium exposure can pose health risks due to its radioactive nature. Inhalation or ingestion of uranium can lead to respiratory problems, kidney damage, and an increased risk of cancer.